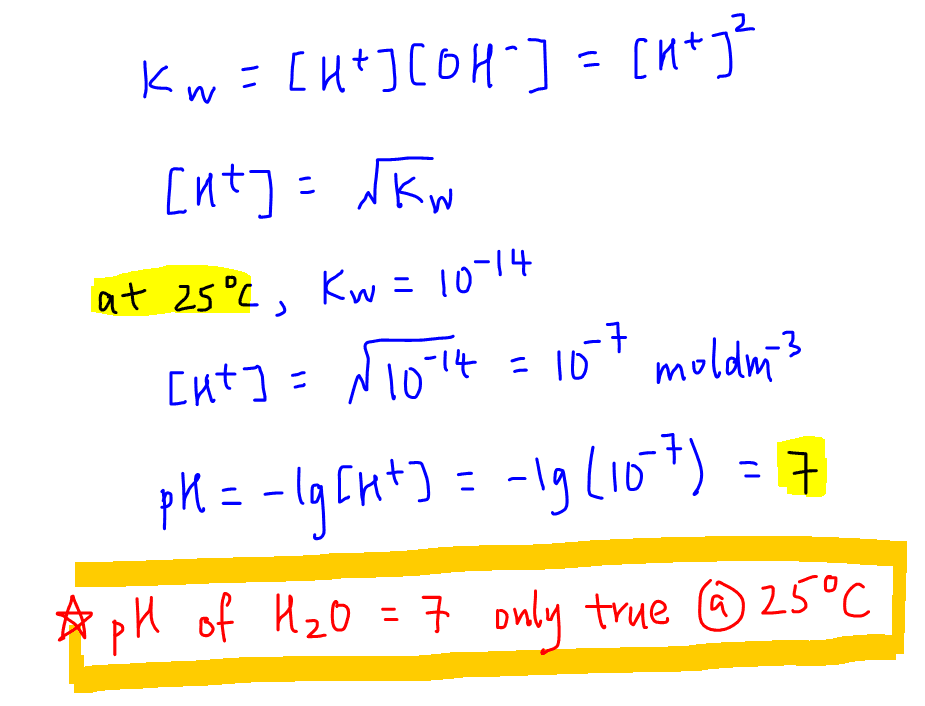“`html
Effective Ways to Calculate pH: Smart Techniques for Chemistry in 2025
The pH, a critical concept in chemistry, biology, and environmental science, represents the acidity or alkalinity of a solution. As we advance into 2025, understanding how to calculate pH using smart techniques will be more important than ever. Whether you are a student, researcher or engaging in chemistry experiments, mastering the pH calculation method will enhance your experiments and analyses. This article guides you through effective ways to measure pH, focusing on various methodologies, tools, and the significance of pH levels.
Understanding the pH Scale
The **pH scale** ranges from 0 to 14 and is a logarithmic measurement of hydrogen ion concentration in a solution. A pH of 7 is considered neutral, while values below this indicate acidity and values above indicate alkalinity. The understanding of pH is crucial in multiple fields including chemistry, biology, and environmental science because it affects chemical reactions, biological processes, and nutrient availability in agriculture. For instance, the **pH of water** impacts aquatic life, soil health, and many biochemical processes. Monitoring the **pH levels** helps in evaluating the quality and safety of water, food products, and soil.
Importance of pH in Different Contexts
The **importance of pH** extends beyond chemistry experiments. In biology, **pH in biology** can influence enzyme activity and metabolic processes. For example, the pH of blood in the human body must be tightly regulated (around 7.4) to maintain physiological functions. Furthermore, understanding **pH in food science** can aid in safe food preservation and ensuring quality control. **Environmental pH testing** can help manage ecosystem health and impact research on climate change.
Measuring pH: Tools and Techniques
Various tools are available for measuring pH. One popular method is using **pH meters**, which provide accurate and immediate readings. Calibration is crucial for precise **lab pH measurement**. Other devices, like **pH test strips**, offer a quick method for on-site measurements, though less precision is expected compared to digital meters. Moreover, chemical indicators can help visually represent pH changes. Universal indicators, phenolphthalein, and litmus paper are common options for qualitative assessments.
Methods for Calculating pH
Understanding the **pH calculation method** is essential. The most common formula to calculate pH is given as:
pH = -log[H⁺]
Where [H⁺] represents the hydrogen ion concentration measured in moles per liter (M). Calculating pH using this formula highlights its logarithmic nature, indicating a tenfold change in hydrogen ion concentration for every unit change in pH. This understanding is crucial for evaluating **strong acids and bases** versus **weak acids and bases** and their respective dissociation behaviors in solutions.
Determining the pH of Solutions
When determining pH, it’s important to consider **solutions of known pH**. This method often involves comparative pH techniques, using standard solutions, which can help calibrate equipment accurately. **Buffer solutions** are fundamental for maintaining a stable pH environment, as they resist changes when acids or bases are added to the solution. Understanding **acidity measurement** allows for effective control of chemical reactions and biological processes.
Real-World Application of pH Calculations
Practically applying pH calculations can be seen in many areas: monitoring **pH in swimming pools** for safe water usage, adjusting **pH in fermentation** processes for optimal growth conditions in brewing, and analyzing **pH in soil** to ensure plants receive necessary nutrients. A good grasp of how to calculate pH can help individuals and businesses optimize various processes.
Using pH Indicators and Titration
Utilizing **pH indicators** throughout experiments increases accuracy and awareness of pH changes. These substances change color based on the solution’s pH, providing a visual representation of acidity or alkalinity. Additionally, **pH titration** is a commonly used laboratory technique that allows chemists to determine the concentration of an acid or a base in a solution accurately. By measuring the volume of titrant added to reach the endpoint where a color change occurs, one can use the data to calculate the original concentration.
Practical Example of Acid-Base Titration
For example, when titrating a strong acid against a strong base, such as HCl with NaOH, using a **pH meter** enables the monitoring of pH changes throughout the process. At the equivalence point (pH of about 7), neutralization occurs, enabling one to calculate the unknown concentration based on the volume used from the titrant. This practical application of pH calculations provides invaluable insights into chemical equilibriums.
Changing pH: Factors and Strategies
Recognizing various factors that can change pH, such as temperature and concentration of solutes, is essential for maintaining optimal conditions. Implementing regular pH checks using **pH testing kits** and **digital pH meters** helps ensure stability in various environments such as laboratories, agricultural fields, and pristine water sources. Moreover, understanding the relationship between **changes in pH** and chemical reactions can lead to better experimental outcomes and refined practices.
Conclusion and Key Takeaways
Understanding how to calculate pH and the implications of pH levels is integral in diverse fields. Keeping track of the pH scale and utilizing proper measurement techniques enriches our knowledge and skills in managing chemical and biological processes. By mastering methods like pH titration and recognizing the importance of buffer solutions, we can ensure stable environments for experiments, agriculture, and health.
Key Takeaways:
- Understanding the pH scale and how to calculate pH are crucial in various fields.
- Utilizing measurement tools like pH meters and testing strips enhances accuracy in pH assessments.
- Application of pH calculations, especially in titration, streamlines experimental research.
- Maintaining steady pH levels is vital for biochemistry, environmental science, and health.
- Regular pH monitoring contributes to better outcomes in both laboratory settings and practical applications.
FAQ
1. What is the best method for measuring pH in the laboratory?
The best method for measuring pH in laboratory settings is through the use of digital pH meters, as they provide precise and quick readings. Regular calibration of these meters enhances their accuracy and reliability during experiments. For those on a budget, pH test strips can offer a simpler, though less precise, alternative.
2. How does temperature affect pH readings?
Temperature can significantly affect pH readings because as temperature increases, the exposure to more kinetic energy alters the ion activity in the solution. This means adjustments often must be made, especially when measuring pH in solutions like water, hence the necessity to always note the temperature during pH assessments.
3. Why is a neutral pH considered important?
A neutral pH is crucial because it represents optimal conditions for many biological and chemical reactions. In biological systems, deviations from this neutral point can negatively impact enzyme activities and cellular functions, making the understanding of neutral pH essential in chemistry and biology.
4. What role does pH play in agriculture?
In agriculture, understanding and managing pH is vital because it affects nutrient availability and plant growth. An ideal pH in soil typically ranges from 6 to 7.5, as this level ensures that vital nutrients are accessible to plants, impacting their overall health and yield.
5. How can I maintain proper pH levels in my swimming pool?
Maintaining proper pH levels in swimming pools requires regular testing, ideally placing the pH between 7.2 and 7.6. This can be managed using chemical additives to increase or decrease pH, alongside maintaining adequate chlorine levels to ensure a safe swimming environment.
6. What are some common pH measurement tools?
Common **pH measurement tools** include digital pH meters, pH test strips, and liquid universal pH indicators. Each tool serves a unique purpose from precision in lab settings to quick, field-based assessments, ensuring the user’s needs are met across diverse environments.
“`