Effective Ways to Name Ionic Compounds
Understanding Ionic Compounds
Ionic compounds are essential in chemistry and are formed through the transfer of electrons between atoms, leading to the creation of charged ions. The ionic bond definition explains that these bonds usually occur between metals and nonmetals. When naming ionic compounds, several principles and guidelines must be adhered to ensure correct chemical nomenclature. In this section, we will discuss the fundamental properties of ionic compounds and their characteristics, which will provide a foundation for understanding naming conventions.
Properties of Ionic Compounds
One of the most distinct characteristics of ionic compounds is their high melting and boiling points due to strong electrostatic forces between the charged ions. These properties arise from the ionic bonds that form between positively charged cations and negatively charged anions. For example, common ionic compounds such as sodium chloride (NaCl) and calcium sulfate (CaSO4) exhibit these properties. Moreover, ionic compounds typically dissolve well in water and conduct electricity in molten or dissolved forms, which makes them unique compared to covalent compounds.
Identifying Ionic Compounds
Understanding how to identify ionic compounds is crucial when applying the naming conventions in chemistry. Ionic compounds typically consist of metal cations and non-metal anions. If you encounter a compound, look for these common characteristics to determine if it is ionic: the presence of metal elements (like sodium, potassium, or calcium) and nonmetals (such as chloride, bromide, or sulfate). Additionally, recognizing polyatomic ions can also help in the identification process, e.g., ammonium (NH4+) and sulfate (SO42−).
Rules for Naming Ionic Compounds
The rules for naming ionic compounds are pivotal to accurately describe ionic substances. These rules encompass systematic approaches that take into account the charge of ions, as well as their classifications, such as transition metals and polyatomic ions. Proper naming is not only essential for clarity in academia but also when discussing the properties and functionalities of these compounds in practical applications.
Binary Ionic Compounds
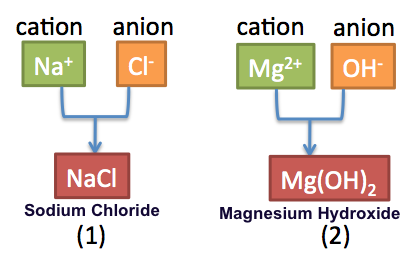
Binary ionic compounds consist of two different elements, typically a metal and a nonmetal. The naming convention for these compounds is straightforward: the name of the cation (the metal) is stated first, followed by the anion (the nonmetal) with its ending changed to “-ide”. For example, potassium bromide follows this pattern, where potassium (K+) is the cation, and bromide (Br−) is the anion. Always remember to verify the charges when dealing with a compound, ensuring overall neutrality.
Naming Transition Metal Compounds
When naming transition metals ionic compounds, it is essential to include the oxidation state of the metal cation in parentheses immediately after its name. This is because transition metals can have multiple oxidation states. For instance, ferrous chloride (FeCl2) signifies iron with a +2 oxidation state, whereas ferric chloride (FeCl3) indicates an iron cation with a +3 charge. Properly denoting the oxidation states prevents confusion and ensures accurate scientific communication.
Common Polyatomic Ions and Their Names
Understanding polyatomic ions is crucial in the systematic naming of ionic compounds. Polyatomic ions are ions made up of multiple atoms that are covalently bonded together but carry a net charge. Recognizing and memorizing the names and charges of these ions is a significant step in mastering compound naming. Their presence can significantly complicate the naming of ionic compounds compared to simple binary combinations.
Common Examples of Polyatomic Ions
Examples of common polyatomic ions include carbonate (CO32−), sulfate (SO42−), and nitrate (NO3−). When naming compounds like calcium carbonate (CaCO3) or sodium sulfate (Na2SO4), the naming conventions follow the established rules, blending the name of the metal with the name of the polyatomic ion. By recognizing these ions and their charges, one can efficiently jot down names of compounds and their formulas.
Nomenclature Rules for Ionic Compounds
Employing proper nomenclature rules for ionic compounds simplifies the learning process. These rules encompass not only how to name ionic substances but also how to write their formulas. When examining stoichiometry, ensure that the total charge balanced by combining cations with anionic charges reflects neutrality. For instance, to form aluminum oxide (Al2O3), electrostatic forces of +3 from aluminum must balance the -2 from oxygen, verifying the formula is correctly composed.
Tips for Effective Naming of Ionic Compounds
Mastering the naming conventions in chemistry can initially seem daunting, yet using a few strategies can simplify the process effectively. Focusing on a systematic naming strategy helps internalize the rules, making them second nature to any chemistry student or practitioner.
Practice with Real-Life Examples
One foundamental tip is to practice with real-life examples of common ionic compounds. Use familiar substances, like sodium chloride (NaCl) or ammonium nitrate (NH4</sub)NO3, and write both their formulas and names. Through continual practice, the comprehension of both naming methods and chemical structures will enhance retention and proficiency. Utilize flashcards, interactive naming games, or quizzes to better learn these concepts for successful naming.
Utilizing Visual Resources
Some learners may find visual resources particularly useful in understanding ionic compound naming conventions. Diagrams that illustrate the formation of ionic bonds and tables that display common cations and anions can reinforce learning and aid memory retention. Incorporating educational tools such as online resources, video tutorials, and infographics can also cater to various learning styles, making complex naming guidelines more accessible.
Conclusion
In summary, effectively naming ionic compounds involves understanding the basic principles behind ionic bonding, adhering to systematic naming rules, and practice with various examples. With these strategies in place, anyone can navigate the complexities of ionic nomenclature with confidence. By utilizing the information and tips shared, you’ll enhance both your essential skills and knowledge in chemistry, positioning yourself for success in the future study and application of ionic compounds.
FAQ
1. What are binary ionic compounds?
Binary ionic compounds are chemical compounds that consist of exactly two different elements—typically a metal (cation) and a nonmetal (anion). When naming binary compounds, the naming conventions dictate that the metal’s name comes first, followed by the anion derived from the nonmetal by altering its ending to “-ide”. Examples include sodium chloride (NaCl) and potassium iodide (KI).
2. How do you name an ionic compound with a polyatomic ion?
To name an ionic compound featuring a polyatomic ion, the process begins with identifying the cation and the polyatomic anion involved. The cation keeps its name, while the polyatomic ion is also referenced as is, without any change needed. For instance, in calcium sulfate (CaSO4), calcium remains unchanged, and sulfate is the name of the polyatomic ion.
3. What is the significance of oxidation states in naming ionic compounds?
Oxidation states are critical in naming ionic compounds, especially for transition metals that have more than one possible charge. Including the oxidation number helps clarify which cation is present, thus preventing ambiguity. For example, copper (II) sulfate (CuSO4) indicates copper with a +2 charge, which distinguishes it from copper (I) sulfate (Cu2SO4), where copper has a +1 charge.
4. Can ionic compounds be formed with nonmetals?
Yes, ionic compounds can be formed with nonmetals, specifically when including polyatomic ions. When nonmetals are paired with metals to form ionic bonds, they typically form negatively charged anions. A classic example is ammonium sulfate, which includes the ammonium ion (NH4+), allowing for ionic bonding with sulfur (SO42−) ions.
5. What are some common mistakes to avoid when naming ionic compounds?
Common mistakes in naming ionic compounds often include failing to determine oxidation states, incorrectly using -ide endings for polyatomic ions, or misrepresenting charged ions. For example, incorrectly calling sodium sulfate “sodium sulfa” disregards standard naming rules. Practice and knowledge of correct ion names help mitigate these errors.
6. How can I better remember polyatomic ions and their names?
To effectively memorize polyatomic ions, mnemonics can help establish relationships or create memorable phrases. Additionally, online games, quizzes, and flashcards are effective tools to reinforce retention. Grouping ions with similar charges or structures enhances memory by recognizing patterns within the nomenclature.